

Ref: 1KT001
Range: 2 to 60°F
Number of tests: 40
Shelf-life : 2 years
The dropper titration method changes the color of the sample from red to blue at the equivalence point. The number of drops needed for the color to change represents the hardness concentration.
Total Hardness (TH) of water is expressed in French degrees (°f), where 1°F corresponds to 10 mg of CaCO₃/L
• Suitable for all types of water
• Meets occasional or domestic use (for frequent testing in an industrial environment, burette methods with large reagent volumes are preferable)
• Option to add your logo
• CMR-free
• Accurate
• Fast and easy-to-use
• Compact and portable
• Affordable


Ref: 70VI0511A
• 86 pre-recorded methods including COD, nitrogen, nitrates, etc., 4 nm bandwidth resolution, optimal precision thanks to the reference beam. Free PC software. Multi-tank accessories, sample suction system and other Quick-lock accessories.

Assess the alkalinity concentration by gradually adding the alkalinity liquor to the sample. Watch the color change from pink to colorless with the TA indicator (CMR-free) or TA phenolphthalein (CMR). For the TAC helianthine the color change is from yellow to orange and from blue-green to pale pink with the TAC reagent.
Learn more
Available in a range of sizes, it is designed to regenerate and disinfect water softener resins. A key step for effective system maintenance.
Learn more
Ref: 1KT005
Range: 0.05 to 2°F
Number of tests: 20 to 40
Ref: ORMCD1003
Range: 1 to 60°F
Number of tests: 20 to 40
Optional cardboard packaging available
Optional 30 ml bottle available
Ref: 1KT004
Range: 2 to 60°F
Number of tests: 40

Ref: 70CI0377 & 70CI0381
• Discover the sturdiness of PRIM LIGHT & ADVANCED. Compact and ultralight. Built-in applications include kinetics and spectral scanning (PRIM Advanced). Free PC software. Integrated calibration filter for reproducible results. Pre-aligned halogen lamp for easy maintenance.

Ref: 70VI0501A
• 86 pre-recorded methods including COD, nitrogen, nitrates, etc., 4 nm bandwidth resolution, optimal precision thanks to the reference beam. Free PC software. Multi-tank accessories, sample suction system and other Quick-Lock accessories.
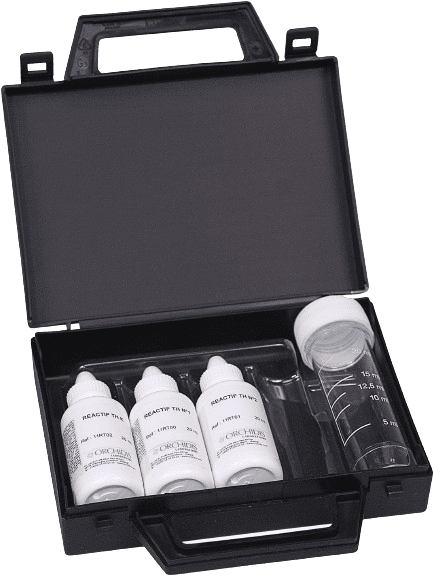
Adjustable hardness test case: dropper, burette of your choice. Additional tests can be included according to your specific needs.
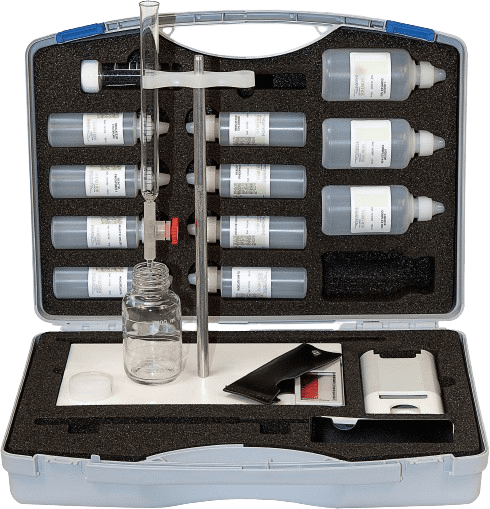
Learn more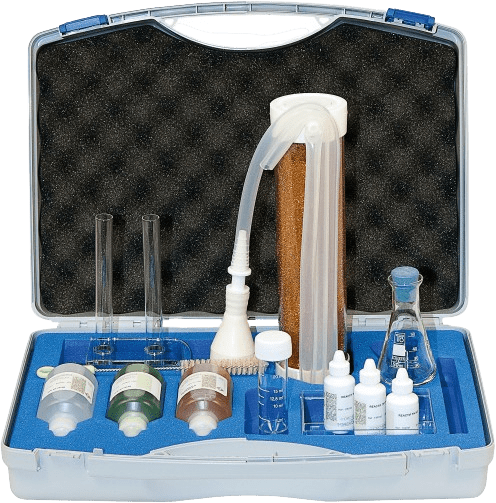
The case contains a mini softener and reagents for hardness, lime precipitation and soap tests. It is ideal for demonstrating the effectiveness of water softeners.
Learn moreAQUALABO, a French company, offers a combination of experience, innovation, international presence, adaptability, and ecological commitment, making it a trusted partner for water quality control.
Measuring hardness is essential in assessing water quality. Hard water can lead to unwanted mineral deposits, affect the taste of drinks, and cause problems with household appliances. Soft water, on the other hand, can be corrosive and requires a delicate balance to prevent damage to your equipment.
Total hardness is the sum of carbonate and non-carbonate hardness. Carbonate hardness, due to calcium and magnesium bicarbonate, is sometimes called temporary hardness, and can be removed by boiling. Noncarbonate hardness, due to calcium and magnesium nitrates, chlorides, and sulfates, is also known as permanent hardness.
Hardness monitoring is vital in many fields, including the food industry, cooling systems, boilers, and power generation. Water of a specific quality, with controlled hardness concentrations, is essential to ensure optimal efficiency of machinery and to extend its useful life. Specific applications include preventing scaling in water heaters, managing boiler water to prevent corrosion, and optimizing the performance of softening systems to eliminate the adverse effects of hardness on industrial processes.
Hardness measures the concentration of calcium and magnesium ions, while alkalinity measures the ability of a solution to neutralize acids. Although related, hardness focuses on calcium and magnesium, while alkalinity includes the contribution of carbonate, bicarbonate, and hydroxide ions.
AQUALABO is a French manufacturer of instrumentation and chemical reagents for the control and analysis of water quality.