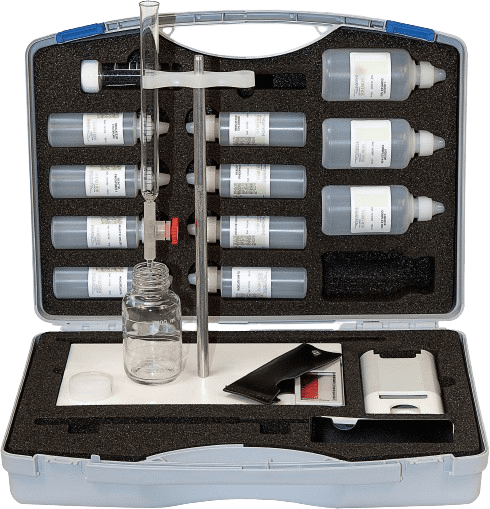
Water hardness test cases
Description
Adjustable hardness test case: dropper, burette of your choice.
Additional tests can be included according to your specific needs. Multi or single parameter analysis cases can accommodate dropper, burette, color scale methods and pocket testers.
Each test case comes with instructions and is fully equipped to carry out the analyses.
Advantages
• Suitable for frequent testing in an industrial environment.
• Suitable for all types of water
Assets
Known for their portability, comprehensiveness, and sturdiness
Similar products
Why trust AQUALABO?
AQUALABO, a French company, offers a combination of experience, innovation, international presence, adaptability, and ecological commitment, making it a trusted partner for water quality control.
FAQ Water hardness test cases
Why measure total hardness?
Measuring hardness is essential in assessing water quality. Hard water can lead to unwanted mineral deposits, affect the taste of drinks, and cause problems with household appliances. Soft water, on the other hand, can be corrosive and requires a delicate balance to prevent damage to your equipment.
What is the difference between carbonated and non-carbonated hardness?
Total hardness is the sum of carbonate and non-carbonate hardness. Carbonate hardness, due to calcium and magnesium bicarbonate, is sometimes called temporary hardness, and can be removed by boiling. Noncarbonate hardness, due to calcium and magnesium nitrates, chlorides, and sulfates, is also known as permanent hardness.
What fields require hardness monitoring?
Hardness monitoring is vital in many fields, including the food industry, cooling systems, boilers, and power generation. Water of a specific quality, with controlled hardness concentrations, is essential to ensure optimal efficiency of machinery and to extend its useful life. Specific applications include preventing scaling in water heaters, managing boiler water to prevent corrosion, and optimizing the performance of softening systems to eliminate the adverse effects of hardness on industrial processes.
What is the difference between hardness and alkalinity?
Hardness measures the concentration of calcium and magnesium ions, while alkalinity measures the ability of a solution to neutralize acids. Although related, hardness focuses on calcium and magnesium, while alkalinity includes the contribution of carbonate, bicarbonate, and hydroxide ions.
